TEST ERA (ENDOMETRIAL RECEPTIVITY ARRAY) IS A MOLECULAR DIAGNOSTIC TEST TO INVESTIGATE THE RECEPTIVITY OF ENDOMETRIUM.
RECEPTIVITY OF THE ENDOMETRIUM IS A CONDITION IN WHICH THE ENDOMETRIUM (UTERINE LINING) IS READY FOR IMPLANTATION (NESTING) OF THE EMBRYO.
Normally, the endometrium is evaluated by using ultrasound that can assess only height and texture of the endometrium. ERA offers an option that increases the likelihood of pregnancy for couples with repeated failure of implantation.
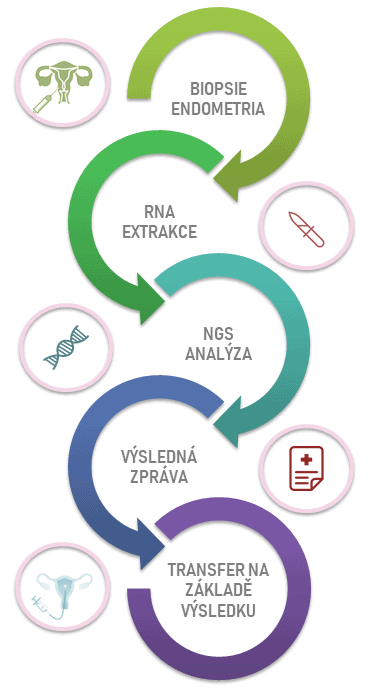
ERA test at the molecular level will determine the expression levels of 238 genes that participate in endometrial receptivity. It will therefore make it possible to evaluate whether the endometrium is properly receptive for embryo transfer in given days or whether the so called “window of implantation” is shifted.
ERA test is carried out optimally during the cycle preceding the one in which the embryo transfer is planned. The tissue for the test is obtained by biopsy of the endometrium. The result will therefore enable us to set the appropriate date of embryo transfer individually for each client.
ERA TEST IS HIGHLY SENSITIVE AND ENABLES DETECTION OF THE RECEPTIVITY OF ENDOMETRIUM AND IS A GOOD CHOICE FOR WOMEN WITH REPEATED UNSUCCESSFUL EMBRYO TRANSFERS.
